
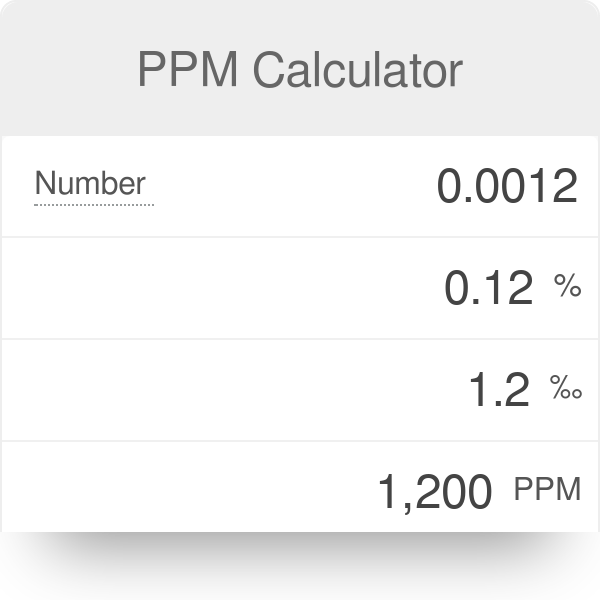
- #How to calculate ppm from grams and percent how to#
- #How to calculate ppm from grams and percent iso#
- #How to calculate ppm from grams and percent free#
#How to calculate ppm from grams and percent iso#
Clean Rooms - ISO Standard 14644 - Clean room class limits according ISO Standard 14644-1.
#How to calculate ppm from grams and percent free#
Clean Rooms - Federal Standard 209 - Clean rooms virtually free of contaminants like dust or bacteria.Apothecaries' Weight System - Apothecaries' fluid and weight system - ounce, drachms, grains, scruples.Ammonia - NH 3 - Concentration in Air and Health Effects - Ammonia and health symptoms - smell and threat to life.Air Contaminants - Limits - Permissible limits to exposure of various air contaminants.Basics - The SI-system, unit converters, physical constants, drawing scales and more.Molality is the number moles of solute divided by kilograms of solvent. Molarity is the number of moles of solute (substance of interest - pollution, etc.) dissolved in one liter (volume) of the solution. Percent by volume = 100 v c /(v c + v s) (3) Volume percent can be expressed as volume per unit volume:

The volume of carbon dioxide in one 1 m 3 of air can be calculated by modifying (1) to The concentration of carbon dioxide in air is aprox. = 8.345 pounds per million gallons of water Example - Volume Concentration of Carbon Dioxide in Air Weight of substance added to one unit volume of water to give one part per million (ppm) The concentration of a component can be measured as mass per unit volume - like mg/liter, mg/cm 3 etc. Weight percent = 100 m c / m s (2) ppm vs.


 0 kommentar(er)
0 kommentar(er)
